Services.
Services.
Technology transfer.
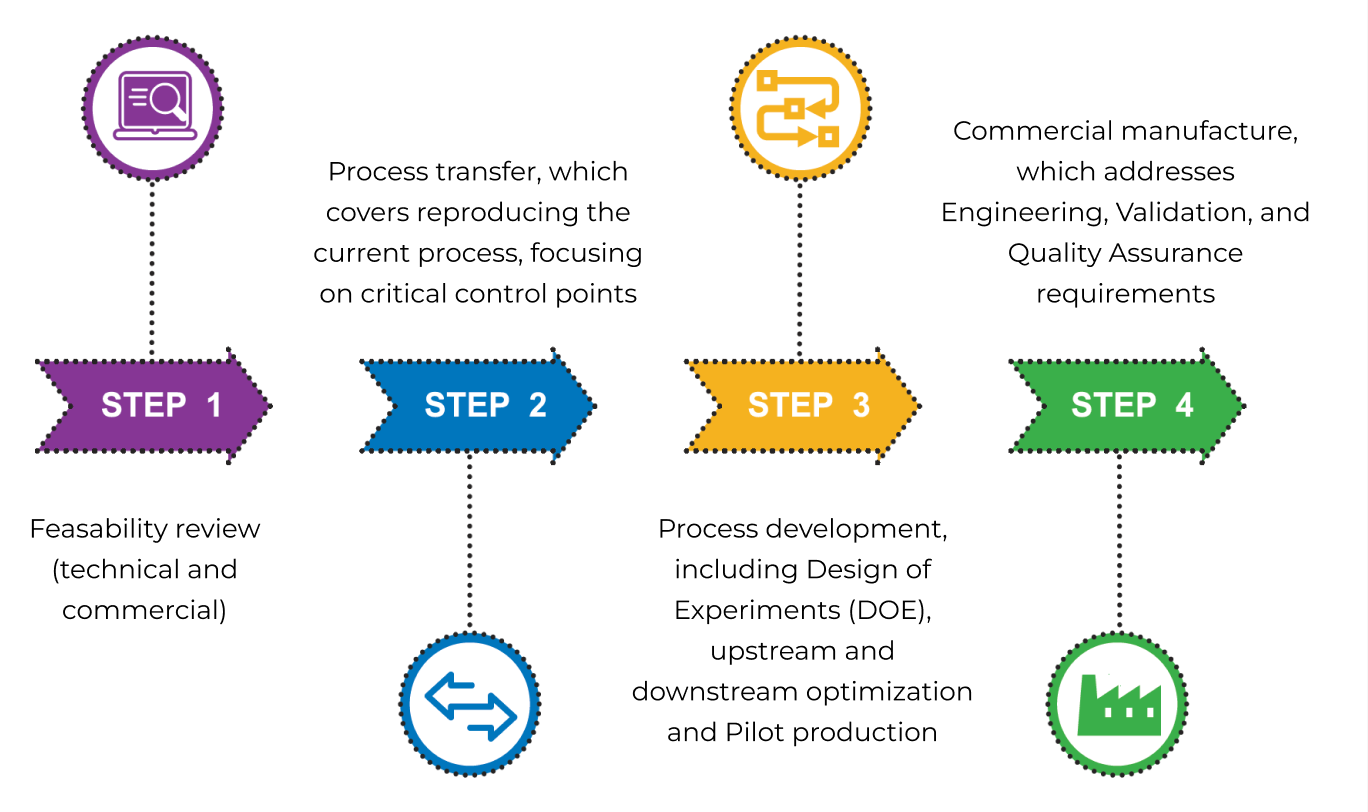
Feasibility review assessment for technical and commercial fit of the process to our facility and equipment.
Process Transfer covers reproducing the current process, focusing on identifying critical control points and parameters. It includes process flow design to identify equipment and process train in facility and analytical alignment.
Process Development includes Design of Experiments (DOE), upstream and downstream optimization, and pilot scale production.
Commercial manufacture follows operational transfer of full-scale production, which addresses Engineering, Validation, and Quality.
Quality Assurance requirements. This covers integration of the process and associated analytical methods into our quality management system (QMS) and process qualification.
Technology transfer.
Feasibility review assessment for technical and commercial fit of the process to our facility and equipment.
Process Transfer covers reproducing the current process, focusing on identifying critical control points and parameters. It includes process flow design to identify equipment and process train in facility and analytical alignment.
Process Development includes Design of Experiments (DOE), upstream and downstream optimization, and pilot scale production.
Commercial manufacture follows operational transfer of full-scale production, which addresses Engineering, Validation, and Quality.
Quality Assurance requirements. This covers integration of the process and associated analytical methods into our quality management system (QMS) and process qualification.
See for yourself.
SEKISUI has vast experience with projects from pre-clinical to commercial, including but not limited to recombinant systems such as Escherichia coli and Pichia pastoris, and associated purification and analytical technologies. Our primary expertise lies in the production of proteins; however, our capabilities are also suitable for plasmids, antibody fragments, DNA modifying enzymes, and other protein production.

