About us.
About us.
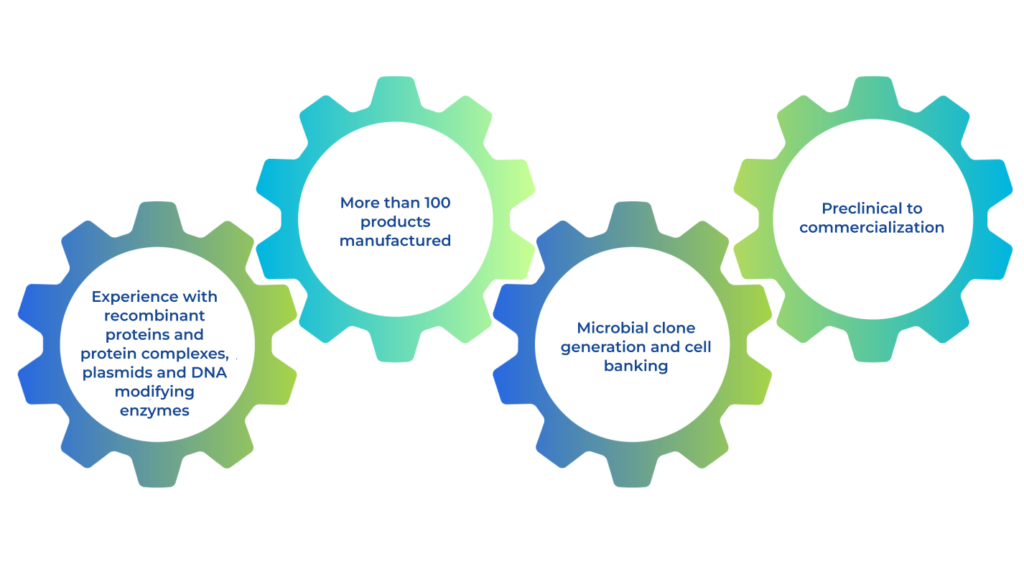
BioProduction by SEKISUI describes SEKISUI’s contract service offering as a fermentation focused CDMO with expertise in proteins and downstream purification. Our microbial process development and production experience helps smooth technical transfer and process scale up. Our core competencies include E.coli and yeast fermentation, plant tissue extraction, large scale bioprocessing, protein purification, bulk formulation, lyophilization and analytical testing.
Our production site in the UK has been a leader in the manufacturing of high-quality proteins and specialty biochemicals globally to the healthcare market for over 40 years. We have worked with many global partners to supply high quality materials for a variety of applications, including proteins for biotherapeutics, pharmaceuticals, and diagnostic medical devices.
BioProduction by SEKISUI describes SEKISUI’s contract service offering as a fermentation focused CDMO with expertise in proteins and downstream purification. Our microbial process development and production experience helps smooth technical transfer and process scale up. Our core competencies include E.coli and yeast fermentation, plant tissue extraction, large scale bioprocessing, protein purification, bulk formulation, lyophilization and analytical testing.
Our production site in the UK has been a leader in the manufacturing of high-quality proteins and specialty biochemicals globally to the healthcare market for over 40 years. We have worked with many global partners to supply high quality materials for a variety of applications, including proteins for biotherapeutics, pharmaceuticals, and diagnostic medical devices.
Learn more about us.

The SEKISUI Advantage
Download the brochure.

Follow us for more.
Visit our LinkedIn.

